Army Pharma Ltd., a state-owned pharmaceutical company, commenced operations in June 2019 under Bangladesh Machine Tools Factory Limited, overseen by the Bangladesh Army. The organization, led by the Chief of Army Staff as Chairman, has three production units focusing on Oral Solid Dosage drugs, parenteral drugs, and a dedicated Cephalosporin unit. The project profile is approved by the Directorate General of Drug Administration, registered with the Registrar of Joint Stock Companies and Firms, and endorsed by the Bangladesh Investment Development Authority. To ensure quality, the company has enlisted the services of Elomatic Consulting & Engineering Ltd, a Finland-based GMP consultant, aiming for cGMP manufacturing licenses and international accreditations.

mission
We prioritize current good manufacturing practice (cGMP) by employing skilled professionals, utilizing quality materials, and employing world-class equipment. Our commitment extends to maintaining rigorous documentation, process validation, and ongoing training, ensuring the consistent delivery of high-quality pharmaceutical products.
Vission
Manufacture and promotion of quality healthcare products for the mankind.
Manufacturing Facilities
Army Pharma Ltd. is dedicated to WHO cGMP guidelines compliance in its manufacturing plant and will supply medicines to the Directorate General of Medical Service and paramilitary institutions. The company sources high-quality raw materials and cGMP-compliant packaging materials from reputable countries, employing a skilled workforce to serve nationwide retail, wholesale markets, and various medical institutions, ensuring top-tier healthcare for all patients in the country.
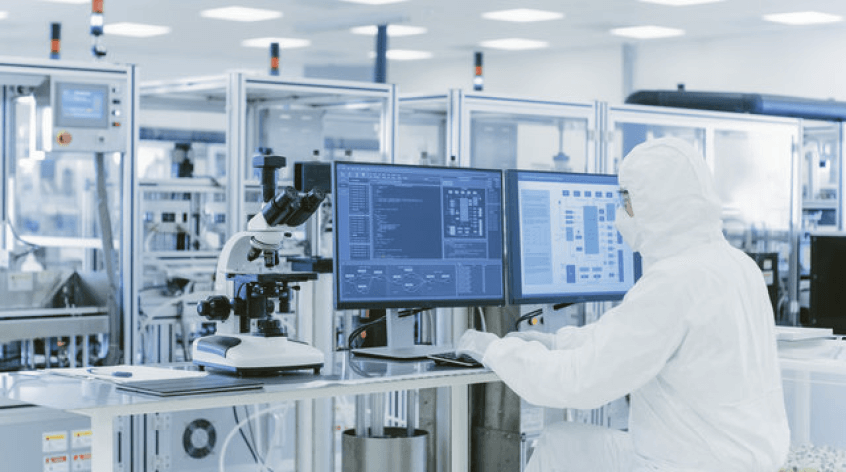
Army Pharma Ltd. is progressing with product development and has established a cutting-edge QC analytical setup, featuring top-tier equipment from global leaders. The company has acquired production machinery from Europe, China, and India, implementing a clean room concept with HVAC systems, sandwich panels, and epoxy floors for cGMP adherence. Stability chambers from Germany, a microbiology lab, and a water treatment plant from Europe augment the facility. The design prioritizes easy cleaning for a hygienic environment, and fire protection measures have been integrated. The comprehensive approach also includes a central BMS for streamlined operations.
Army Pharma Ltd. prioritizes sustainability and quality, evident in its waste water disposal and Zero Liquid Discharge (ZLD) system. The company integrates advanced technologies, including heavy metal removal, green initiatives, and safety measures like incineration and animal shelters. Unidirectional material flow with In-process Quality Control (IPQC) ensures efficiency, while independent quality monitoring and serialization combat counterfeits. Adhering to International Conference on Harmonization (ICH) guidelines, products undergo stability studies before release. The company fosters an eco-friendly environment with facilities like a medical center, laundry, fire unit, and water bodies.

Products and product line
Bangladesh Army Pharma Ltd. has strategically planned its site to accommodate Anti-cancer, Hormone, Vaccine, Biotec, Agrovet, Herbal, and Medical Device plants. Market experts have meticulously selected 300 dynamic molecules through Intercontinental Medical Statistics (IMS), emphasizing high market share and growth. The company will manufacture a diverse range of pharmaceuticals, including tablets, capsules, syrups, injectable, creams, and more, covering therapeutic subclasses such as Antibiotics, Cardiovascular drugs, Neurological products, and Geriatric medicines. Prioritizing institutional, domestic, wholesale, and international markets, Army Pharma Ltd. is dedicated to producing new generics and biosimilar, exemplified by its already developed products like Paracetamol and Esomeprazole tablets, showcasing a commitment to innovation in the pharmaceutical sector.
Healthcare and Hygiene
Under the guidance of Army Pharma Limited, Bangladesh Machine Tools Factory Ltd. (BMTF) is actively engaged in the manufacturing and retailing of Healthcare and Hygiene products. BMTF holds a No Objection Certificate (NOC) from the Directorate General of Drug Administration (DGDA) and is certified by the Bangladesh Standards and Testing Institution (BSTI). With a presence in retail, wholesale, outlets, and institutions, BMTF plays a vital role in providing quality healthcare and hygiene solutions in Bangladesh, maintaining strict adherence to regulatory standards.
Packaging Factory
We are producing pharmaceutical primary packaging materials with the only virgin grade raw materials in our factory. The vision of the packaging factory is to provide the finest quality of finished product in your hand.

Our Aim
Use class D environment as per Pharmaceutical clean room concept for finished goods packing
Use only virgin grade resin
Skilled and experienced workforce
Affordable price

Product Categories
We can serve you by offering the following product categories
Health & Hygiene
Packaging Factory
Beauty Products
Animal Health Care Products
Animal Health
In January 2023, BMTF Ltd. proudly introduced a line of animal healthcare products, underscoring our commitment to quality and promotion. Sourcing raw materials from validated channels, we adhere to the stringent guidelines of the Directorate General of Drug Administration (DGDA) and have established a world-class QC lab. With approval from the Directorate of Livestock Services (DLS), our State-of-The-Art production facilities operate under the supervision of a qualified multidisciplinary team comprising Veterinary Doctors, Pharmacists, Biochemists, Chemists, and Microbiologists. Embracing Current Good Manufacturing Practice (cGMP) standards, including a clean room concept and unidirectional production lines, we ensure product quality. Each production batch is released only after receiving a finished goods qualification certificate from our meticulous Quality Assurance (QA) department.